Polyclonal antibody to Caspase-14

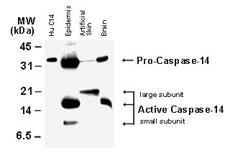
Fig:1 Western blot analysis of Caspase-14. Tissue lysates (50 ug/lane) and recombinant human Caspase-14 were (Hu C14, 15 ng) were Fig:1 Western blotted with Caspase-14 antibody (20-1052) at 1:2000. The antibody detected both the proform of caspase-14, and the large and small subunits of active/cleaved caspase-14.
Roll over image to zoom in
Shipping Info:
Order now and get it on Wednesday April 23, 2025
Same day delivery FREE on San Diego area orders placed by 1.00 PM
| Format : | Sera |
| Amount : | 50 µl |
| Isotype : | Rabbit IgG |
| Content : | 50 µl sera |
| Storage condition : | Store the antibody at 4°C, stable for 6 months. For long-term storage, store at -20°C. Avoid repeated freeze and thaw cycles. |
Apoptosis, or programmed cell death, is a common property of all multicellular organisms. The current dogma of apoptosis suggests that the components of the core celldeath machinery are integral to cells and widely conserved across species. Caspases are typically divided into 3 major groups, depending on the structure of their prodomain and their function. Group 1: inflammatory caspases (caspases 1, 4, 5, 11, 12, 14). Group II: initiator of apoptosis caspases (2, 8, 9). Group III: effector caspases (3, 6, 7). Caspases are constitutively expressed in almost all cell types as inactive proenzymes (zymogens: enzyme precursors which require a biochemical change to become active enzymes) that are processed and activated in response to a variety of pro-apoptotic or inflammatory stimuli. The procaspases (32-56 kDa) contain four domains: an N-terminal prodomain (2-25 kDa), a large subunit (p20: 17-21 kDa), a small subunit (p10: 10-13 kDa) and a short linker region between the large and small subunits. Caspase activation involves proteolytic processing of the proenzyme at specific aspartate residues between the domains. This results in removal of the prodomain as well as the linker region and formation of a heterodimer containing one large and one small subunit (p20-p10). Active caspases mediate cell death and inflammation through cleavage of particular cellular substrates that are involved in these processes. Caspase-14 activation has been implicated in keratinocyte senescence which leads to the cornified cell layer, suggesting a role for caspase-14 in epithelial cell differentiation. Tumor-specific alterations in caspase-14 expression have been found in epithelial malignancies, suggesting that caspase-14 plays a role in epithelial cell transformation. This antibody recognizes the proform of caspase-14 (~28-32 kDa), and the large (~14-21 kDa) and small (~10-11 kDa) of active/cleaved caspase-14.
WB: 1:1000-1:2000, IHC (paraffin): 1:1000-1:5000, IHC (frozen): Users should optimize, IP: 1:50-1:200
For Research Use Only. Not for use in diagnostic/therapeutics procedures.
| Subcellular location: | Cytoplasm, Nucleus |
| Post transnational modification: | Maturation by proteolytic processing appears to be a two-step process. The precursor is processed by KLK7 to yield the p20/p8 intermediate form which acts on the precursor to yield the p17/p10 mature form (PubMed:22825846). Initially, cleavage between Ile-152 and Lys-153 has been proposed to yield the large and small subunits of the active enzyme (PubMed:12200134). |
| Tissue Specificity: | Expressed in keratinocytes of adult skin suprabasal layers (from spinous layers to the stratum granulosum and stratum corneum) (at protein level). Expressed in keratinocytes of hair shaft and sebaceous glands (at protein level). In psoriatic skin only expressed at very low levels (PubMed:11175259). The p17/10 mature form is expressed in epidermis stratum corneum, the p20/p8 intermediate form in epidermis upper granular cells of the stratum granulosum (PubMed:22825846). |
| BioGrid: | 117116. 33 interactions. |
|
There are currently no product reviews
|



















.png)











