Human GM-CSF / CSF2 Recombinant Protein (Fc Tag)(Discontinued)
Shipping Info:
For estimated delivery dates, please contact us at [email protected]
| Amount : | 20 µg |
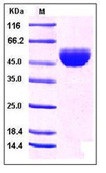
| Purification : | > 97 % as determined by SDS-PAGE |
| Content : | Formulation Lyophilized from sterile 100mM Glycine, 10mM NaCl, 50mM Tris, pH 7.5 Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Storage condition : | Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| AA sequence : | Ala18-Glu144 |
| Alternative Name : | CSF2 Protein, GM-CSF Protein, GMCSF Protein, |
Source : HEK293 Cells
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is one of an array of cytokines with pivotal roles in embryo implantation and subsequent development. Several cell lineages in the reproductive tract and gestational tissues synthesise GM-CSF under direction by ovarian steroid hormones and signalling agents originating in male seminal fluid and the conceptus. The pre-implantation embryo, invading placental trophoblast cells and the abundant populations of leukocytes controlling maternal immune tolerance are all subject to GM-CSF regulation. GM-CSF stimulates the differentiation of hematopoietic progenitors to monocytes and neutrophils, and reduces the risk for febrile neutropenia in cancer patients. GM-CSF also has been shown to induce the differentiation of myeloid dendritic cells (DCs) that promote the development of T-helper type 1 (cellular) immune responses in cognate T cells. The active form of the protein is found extracellularly as a homodimer, and the encoding gene is localized to a related gene cluster at chromosome region 5q31 which is known to be associated with 5q-syndrome and acute myelogenous leukemia. As a part of the immune/inflammatory cascade, GM-CSF promotes Th1 biased immune response, angiogenesis, allergic inflammation, and the development of autoimmunity, and thus worthy of consideration for therapeutic target. GM-CSF has been utilized in the clinical management of multiple disease processes. Most recently, GM-CSF has been incorporated into the treatment of malignancies as a sole therapy, as well as a vaccine adjuvant. While the benefits of GM-CSF in this arena have been promising, recent reports have suggested the potential for GM-CSF to induce immune suppression and, thus, negatively impact outcomes in the management of cancer patients. GM-CSF deficiency in pregnancy adversely impacts fetal and placental development, as well as progeny viability and growth after birth, highlighting this cytokine as a central maternal determinant of pregnancy outcome with clinical relevance in human fertility. Cancer Immunotherapy Immune Checkpoint Immunotherapy Targeted Therapy
1. Measured by its binding ability in a functional ELISA. Immobilized CD131 at 10 µg/ml (100 µL/well) can bind recombinant human GM-CSF / Fc with a linear range of 0.032-4 µg/ml. 2. Measured in a cell proliferation assay using TF-1 human erythroleukemic cells. The ED50 for this effect is typically 1-5 ng/mL.
Endotoxin :< 1.0 EU per µg of the protein as determined by the LAL method.
Other pack size also available.
For Research Use Only. Not for use in diagnostic/therapeutics procedures.
|
There are currently no product reviews
|














.png)








