Human Carboxypeptidase A1 / CPA1 Recombinant Protein (His Tag)(Discontinued)
Shipping Info:
For estimated delivery dates, please contact us at [email protected]
| Amount : | 20 µg |
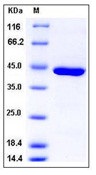
| Purification : | > 97 % as determined by SDS-PAGE |
| Content : | Formulation Lyophilized from sterile PBS, pH 7.4 Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Storage condition : | Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| AA sequence : | Met1-Tyr419 |
| Alternative Name : | CPA Protein, |
Source : HEK293 Cells
Carboxypeptidase A1 (CPA1)is secreted as a pancreatic procarboxypeptidase, and cleaves the C-terminal amide or ester bond of peptides that have a free C-terminal carboxyl group, with the preference of residues with aromatic or branched aliphatic side chains. CPA1 comprises a signal peptide, a pro region and a mature chain, and can be activated after cleavage of the pro peptide. In contrast to procarboxypeptidase B which was always secreted by the pancreas as a monomer, procarboxypeptidase A occurs as a monomer and/or associated to one or two functionally different proteins, such as zymogen E, and is involved in zymogen inhibition. Three different forms of human pancreatic procarboxypeptidase A have been isolated.
Carboxypeptidase A1 (CPA1)is secreted as a pancreatic procarboxypeptidase, and cleaves the C-terminal amide or ester bond of peptides that have a free C-terminal carboxyl group, with the preference of residues with aromatic or branched aliphatic side chains. CPA1 comprises a signal peptide, a pro region and a mature chain, and can be activated after cleavage of the pro peptide. In contrast to procarboxypeptidase B which was always secreted by the pancreas as a monomer, procarboxypeptidase A occurs as a monomer and/or associated to one or two functionally different proteins, such as zymogen E, and is involved in zymogen inhibition. Three different forms of human pancreatic procarboxypeptidase A have been isolated.
Measured by its ability to cleave the colorimetric peptide substrate Ac-Phe-Thiaphe-OH in the presence of 5,5'Dithiobis (2-nitrobenzoic acid) (DTNB). The specific activity is >3,500 pmoles/min/µg .
Endotoxin :< 1.0 EU per µg of the protein as determined by the LAL method
For Research Use Only. Not for use in diagnostic/therapeutics procedures.
|
There are currently no product reviews
|
















.png)










