Anti-Syk Monoclonal Antibody (Clone:SYK-01)

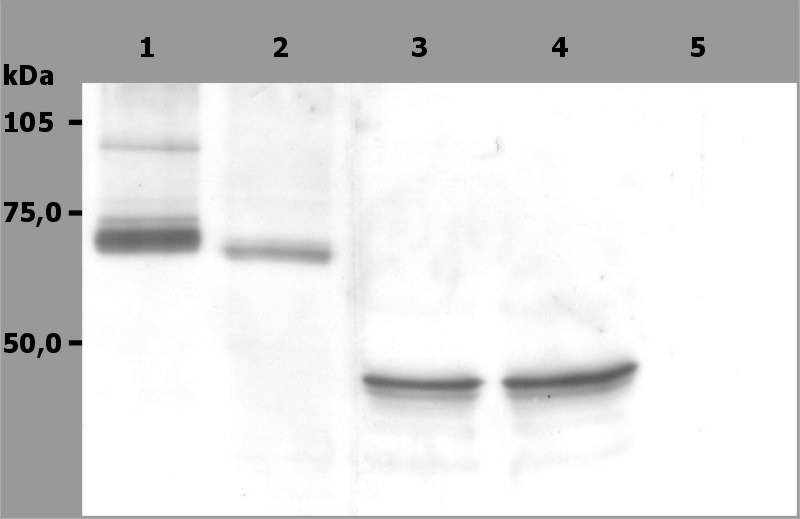
Figure 1: Western Blotting analysis (non-reducing conditions) of whole cell lysate of RAMOS human Burkitt lymphoma cell line (1), RBL rat basophilic leukemia cell line (2) and HeLa human cervix carcinoma cell line (3, 4). Lane 2: immunostaining with anti-Syk (SYK-01). Lane 3, 4: immunostaining with anti-human Cytokeratin 18 (DC-10; ). Lane 5: negative control
Roll over image to zoom in
Shipping Info:
For estimated delivery dates, please contact us at [email protected]
| Format : | Purified |
| Amount : | 0.1 mg |
| Isotype : | Mouse IgG1 |
| Purification : | Purified by protein-A affinity chromatography |
| Storage condition : | Store at 2-8°C. Do not freeze. |
Syk is a cytoplasmic protein tyrosine kinase that translocates to the plasma membrane upon B cell antigen receptor (BCR) or the high-affinity IgE receptor (FcepsilonRI) triggering, and phosphorylates downstream adaptor proteins, thereby providing docking sites for initiation of subsequent signaling pathways, such as calcium mobilization, cytoskeleton remodeling, or transcription of specific genes. Syk binds to the receptor assemblies through interactions of its pair of SH2 domains with ITAM motives of the receptor, which have been phosphorylated by Src-family kinases. These kinases also help to activate Syk by phosphorylation of its activation loop.
Immunofluoroscence Western Blotting Recommended dilution:
1-2 µg/ml, 60 min
Positive control:
RBL rat basophilic leukemia cell line
A-431 human epidermoid carcinoma cell line
RAMOS lymphoma cell line
U-937 human histiocytic lymphoma cell line
JURKAT human peripheral blood T cell leukemia cell line
Negative control:
HeLa human cervix carcinoma cell line
For Research Use Only. Not for use in diagnostic/therapeutics procedures.
| Subcellular location: | Cell membrane, Cytoplasm |
| Post transnational modification: | Autophosphorylated. Phosphorylated on tyrosine residues by LYN following receptors engagement. Phosphorylation on Tyr-323 creates a binding site for CBL, an adapter protein that serves as a negative regulator of BCR-stimulated calcium ion signaling. Phosphorylation at Tyr-348 creates a binding site for VAV1. Phosphorylation on Tyr-348 and Tyr-352 enhances the phosphorylation and activation of phospholipase C-gamma and the early phase of calcium ion mobilization via a phosphoinositide 3-kinase-independent pathway (By similarity). Phosphorylated on tyrosine residues in response to IL15 (PubMed:15123770). Phosphorylation on Ser-297 is very common, it peaks 5 minutes after BCR stimulation, and creates a binding site for YWHAG. Phosphorylation at Tyr-630 creates a binding site for BLNK. Dephosphorylated by PTPN6. |
| Tissue Specificity: | Widely expressed in hematopoietic cells (at protein level) (PubMed:8163536). Expressed in neutrophils (at protein level) (PubMed:15123770). Within the B-cell compartment, expressed from pro- and pre-B cells to plasma cells (PubMed:8163536). |
| BioGrid: | 112717. 111 interactions. |
|
There are currently no product reviews
|
















.png)











