Anti-HSP70/HSC70 Monoclonal Antibody (Clone : N27F3-4) ATTO 594

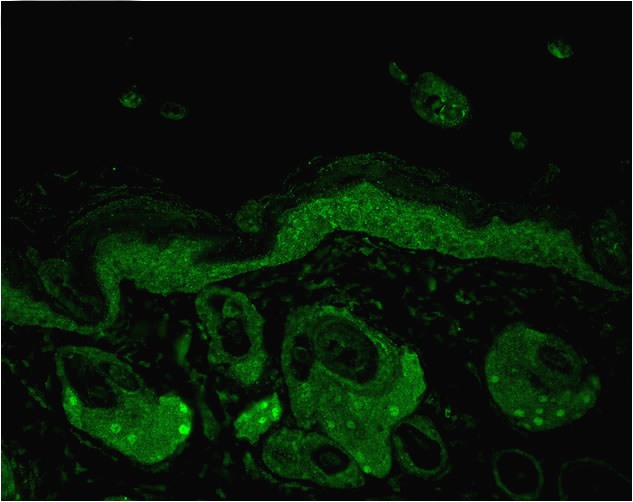
Figure1 : Mouse Anti-Hsp70 Antibody[N27F3-4] used in Immunohistochemistry (IHC) on Mouse backskin
Roll over image to zoom in
Shipping Info:
For estimated delivery dates, please contact us at [email protected]
| Amount : | 200 µg |
| Isotype : | Mouse IgG1 |
| Purification : | Protein G Purified |
| Content : | PBS pH7.2, 50% glycerol, 0.09% sodium azide |
| Storage condition : | Store the antibody at 4°C |
HSP70 genes encode abundant heat-inducible 70-kDa HSPs (HSP70s). In most eukaryotes HSP70 genes exist as part of a multigene family. They are found in most cellular compartments of eukaryotes including nuclei, mitochondria, chloroplasts, the endoplasmic reticulum and the cytosol, as well as in bacteria. The genes show a high degree of conservation, having at least 50% identity. The N-terminal two thirds of HSP70s are more conserved than the C-terminal third. HSP70 binds ATP with high affinity and possesses a weak ATPase activity which can be stimulated by binding to unfolded proteins and synthetic peptides. When HSC70 (constitutively expressed) present in mammalian cells was truncated, ATP binding activity was found to reside in an N-terminal fragment of 44 kDa which lacked peptide binding capacity. Polypeptide binding ability therefore resided within the C-terminal half. The structure of this ATP binding domain displays multiple features of nucleotide binding proteins. All HSP70s, regardless of location, bind proteins, particularly unfolded ones. The molecular chaperones of the HSP70 family recognize and bind to nascent polypeptide chains as well as partially folded intermediates of proteins preventing their aggregation and misfolding. The binding of ATP triggers a critical conformational change leading to the release of the bound substrate protein. The universal ability of HSP70s to undergo cycles of binding to and release from hydrophobic stretches of partially unfolded proteins determines their role in a great variety of vital intracellular functions such as protein synthesis, protein folding and oligomerization and protein transport.
WB (1:1000), IHC (1:100), ICC/IF (1:50); optimal dilutions for assays should be determined by the user.
For Research Use Only. Not for use in diagnostic/therapeutics procedures.
| Subcellular location: | Cytoplasm, Nucleus, Cytoplasm |
| Post transnational modification: | In response to cellular stress, acetylated at Lys-77 by NA110 and then gradually deacetylated by HDAC4 at later stages. Acetylation enhances its chaperone activity and also determines whether it will function as a chaperone for protein refolding or degradation by controlling its binding to co-chaperones HOPX and STUB1. The acetylated form and the non-acetylated form bind to HOPX and STUB1 respectively. Acetylation also protects cells against various types of cellular stress. |
|
There are currently no product reviews
|




















.png)












